![]() In 1800 only 3% of the world’s population lived in urban areas, yet as the industrial revolution picked up pace in the early 18th and 19th centuries the number of people moving from the countryside to work in the newly industrialised cities soared. By 1950 29% of the world’s population were living in cities and by 1985 this had grown to 42% while in 2025 it is estimated to that it will be over 60%1. That is just 10 years from now. In highly developed nations such as those in Western Europe and North America the 50% threshold has already been surpassed which means that if you live in a western nation and do not live in a city you are in a minority.
In 1800 only 3% of the world’s population lived in urban areas, yet as the industrial revolution picked up pace in the early 18th and 19th centuries the number of people moving from the countryside to work in the newly industrialised cities soared. By 1950 29% of the world’s population were living in cities and by 1985 this had grown to 42% while in 2025 it is estimated to that it will be over 60%1. That is just 10 years from now. In highly developed nations such as those in Western Europe and North America the 50% threshold has already been surpassed which means that if you live in a western nation and do not live in a city you are in a minority.

It’s not just where people choose to live that is rapidly changing either, but how many of us there are. The human population is currently expanding more rapidly than ever before and has grown from just a few hundred million people less than a thousand years ago to over 7 billion today, and this number is still rising2. As the human population grows cities are expanding quickly to meet the need for additional housing while the surrounding countryside is farmed and developed ever more intensely to provide us with the food, water and other resources we need to live.
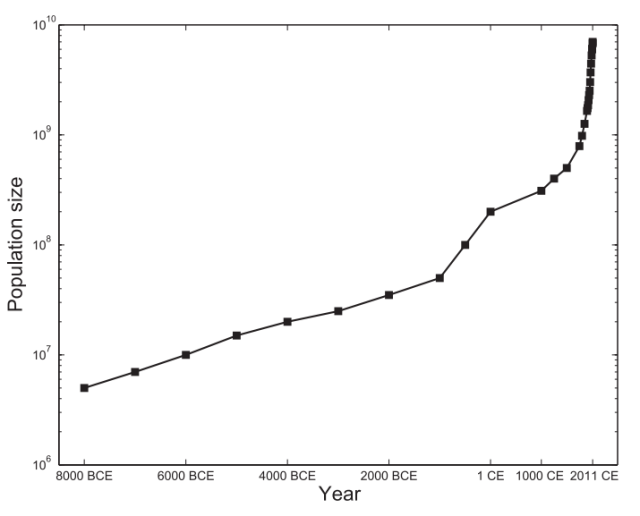
Naturally, many people are concerned about how we are going to continue to feed and clothe ourselves as populations continue to expand, but there is also a growing concern among many about how such huge numbers of people are affecting the environment and the animals that inhabit it.
For animals which depend on natural habitats such as forests, meadows, or wetlands to survive, the growth of urban areas often spells bad news as these habitats are removed and paved over to make way for new suburbs, factories and roads. In the UK numerous species have declined for just this reason. For example, the bittern, a close relative of herons, was once widespread in reedbeds and wetlands across the UK but is now confined to a tiny area of the south-east after its habitats were drained to make way for agriculture and urban developments.
While many species cannot survive in urbanised areas, others are able to tolerate moderate levels of urbanisation and may continue living within cities despite drastic changes to their habitats. Life in the city is not without its challenges however, even for the most adaptable and resilient of species. Cities typically contain different threats to rural areas such as an abundance of cats which are responsible for killing huge numbers of birds and small mammals4, and high levels of chemical5, light6 and noise pollution7 which all have negative impacts.
This all sounds bad, and it really is, but while many species suffer badly from the effects of increasing urbanisation and habitat loss, there are a few species that have been able to adjust remarkably well to life in urban areas. One group that has been particularly well-studied in this regard are the songbirds and over the past 15 years or so, biologists have discovered some fascinating behavioural adaptations which have allowed some species to become successful city dwellers.

One of the most notable features of cities across the world is that they are incredibly noisy places. With heavy traffic, building sites, aircraft flying overhead and all manner of other sounds and distractions it’s a miracle anyone can hear anything at all. For songbirds however, all this noise is more than just a distraction, it can seriously affect their chances of finding mates and successfully reproducing and for males it is likely to affect how well they can defend their territories against rivals.
While we may find bird song pleasant to listen to (or annoying depending on how early in the morning it is), for songbirds it has a serious purpose. Males sing during the breeding season to attract females8 and to signal to other males that their territory is occupied and should not be entered9.
City noise can overlap and interfere with these signals making communication among birds difficult and unreliable. The background noise of a city is typically continuous low rumble concentrated at around 2kHz in frequency. Unfortunately for many birds this overlaps neatly with the frequency of their songs and this can make it difficult for other birds to hear them as they do not stand out from the irrelevant background noise.
Clearly this is a problem for birds which rely on song to communicate, yet research has revealed that birds have ways of overcoming this problem and one of them is to increase the frequency at which they sing so that their songs literally rise above the background noise and can be clearly heard.
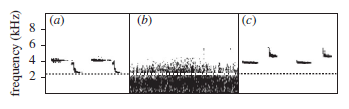
Evidence that birds sing at higher frequencies in noisy cities than they do in quieter rural sites has now been found in numerous species including great tits (Parus major)7, blackbirds (Turdus merula)10, European robins (Erithacus rubecula)11 and song sparrows (Melospiza melodia)12.
In great tits the difference in song frequency between urban and rural sites has been measured at 478Hz13 and tests have shown that this is enough to substantially improve the distance over which song can travel in urban environments before it degrades and becomes inaudible14.
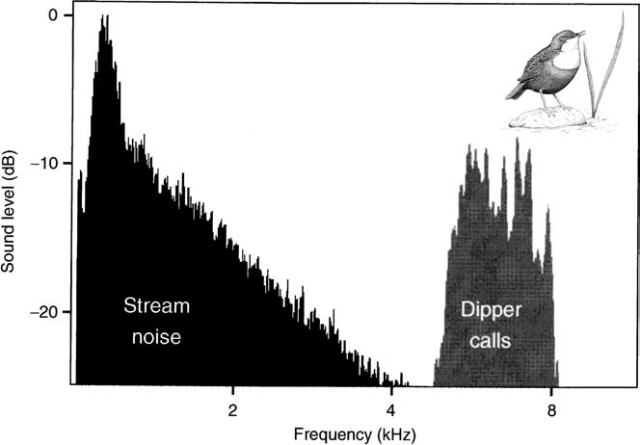
Birds may also face challenging noisy conditions in natural environments too such as where running water or wind creates high levels of low-frequency noise and these naturally noisy sites have allowed scientists to confirm that it really is the noise in cities and not some other factor which is causing city birds to sing at high frequencies. Biologists Henrik Brumm and Hans Slabbekoorn recorded the songs of white-throated dippers (Cinclus cinclus) living around noisy fast flowing streams in Scotland and found that they call at frequencies well above that of the background noise and higher than usual for this species suggesting that dippers in this area have adapted their calls to suit their noisy habitat15.
The effect of natural background noise on song frequency has also been shown in African little greenbuls (Andropadus virens) which sing at a higher frequency in areas where the rainforest is merging with open grasslands (known as ecotone forests) than they do deep within the rainforest itself16. Analysis of these two habitats revealed that the background noise in the rainforest is largely concentrated at higher frequencies while in ecotone forests there is more low-frequency noise. By singing at a lower frequency little greenbuls within the rainforest can ensure that their song does not overlap with the higher frequency background noise found in rainforests, while by singing at a lower frequency little greenbuls in ecotone forests avoid the lower frequency background noise in their habitat.
The evidence that birds change the frequency of their songs as an adaptation to noisy conditions may seem quite conclusive but not everyone agrees. An alternative explanation for the observed frequency shifts is that higher frequency song is actually just an unavoidable and possibly unimportant side-effect of singing more loudly, and it is higher volume, not frequency, which allows birds in noisy environments to overcome the background noise17.
In support of this argument Erwin Nemeth and Henrik Brumm of the Max Planck Institute for Ornithology in Germany found that the typical increases in song frequency found in great tits and blackbirds may be too low to substantially improve signal transmission whereas small increases in song amplitude were found to increase the distance over which a bird’s song could be detected much more effectively18.
But why should song frequency increase when birds sing more loudly? Nemeth and Brumm suggest two possibilities. Firstly, the increase in frequency observed in songs in noisy environments could be a side-effect of what is known as the Lombard effect (named after the French scientist Étienne Lombard) in which animals unconsciously increase the volume and frequency of their calls when the level of the background noise rises.
![Nachtigall_%28Luscinia_megarhynchos%29-2[1]](https://ecologicablog.files.wordpress.com/2015/02/nachtigall_28luscinia_megarhynchos29-21.jpg?w=640)
The Lombard effect is known to occur in humans (this is why it might feel like you have to shout to be heard at loud parties) and has also been shown in both lab and field studies of songbirds. Lab experiments on elegant crested tinamous (Eudromia elegans)19, and budgerigars (Melopsittacus undulates)20, have shown that these species both sing more loudly and at a higher frequency when background noise increases and the same result has been shown in the field in a study of Nightingales (Luscinia megarhynchos) in Berlin21.
A second reason why song frequency may increase when birds sing more loudly is that both the volume and frequency of bird songs depend on the same song producing organ which could limit how well birds can independently control frequency and volume. In birds this organ is the syrinx which is the bird equivalent of the mammalian larynx or voice box and is located at the base of the windpipe connected to the lungs. Birds produce song by forcing air at high pressure from the lungs through the syrinx causing membranes to vibrate creating sound. This sound can then be modified using numerous tiny muscles which alter the shape and tension of the sound producing membranes.
However, past studies of the avian vocal system have shown that without these tiny muscles altering the structure of the sound, both the frequency and amplitude of bird song unavoidably increase together. In other words, when birds sing louder they cannot help but also sing at a higher frequency22.
Of course, this may be totally irrelevant if birds are able to use muscles to independently control the frequency and volume of their songs but there is evidence to suggest that the frequency and volume of bird songs really are closely intertwined. One of the clearest examples of this comes from a study by Nemeth and his colleagues at the Max Planck institute who recorded blackbirds singing in sound-proof chambers and showed that volume and frequency really were strongly correlated17. When blackbirds sing more loudly they also sing at a higher frequency and this may be totally involuntary. Similar results have been found in other species including zebra finches (Taeniopygia guttata)23 and song doves (Streptopelia risoria)24 suggesting that this pattern may be widespread in birds as a whole.
![1280px-Turdus_merula_-Gran_Canaria%2C_Canary_Islands%2C_Spain-8_%282%29[1]](https://ecologicablog.files.wordpress.com/2015/02/1280px-turdus_merula_-gran_canaria2c_canary_islands2c_spain-8_282291.jpg?w=483&h=362)
It has become very clear over the past few years that urban noise is causing bird song to change however, opinion is still divided on whether it is the frequency or amplitude changes that are most important to improving song transmission in noisy environments. It is possible that both have important roles to play in helping birds to adapt to noisy urban areas and hopefully future research will provide an answer to this question.
The study of how urban noise affects bird song is a very active area of research and there are many unresolved questions which are likely to be answered in the next few years. Most importantly we need to find out what the long-term impacts of urban noise are on bird populations. Although many species of birds do seem to be able to adapt to noise we do not know how the dramatic changes we are causing to their environments will affect them in the long-term. Furthermore, many species are not able to adapt to urban areas for numerous possible reasons. They may not possess the behavioural flexibility to cope with new environments or not they might not be physiologically capable of changes their songs or behaviour. That is why studies those discussed here matter, we are changing the planet in ways which have never been seen before and we know that many species are suffering as a result. The first step to protecting animals from these changes is to understand how they are affected and that is just what these studies aim to do.
References
1. Kegel, B (2014).Tiere in der Stadt: Eine Naturgeschichte. Köln: DuMont Buchverlag. (In German).
2. Keinan, A., & Clark, A. (2012). Recent Explosive Human Population Growth Has Resulted in an Excess of Rare Genetic Variants. Science, 336 (6082), 740-743 DOI: 10.1126/science.1217283
3. Barnosky AD, Matzke N, Tomiya S, Wogan GO, Swartz B, Quental TB, Marshall C, McGuire JL, Lindsey EL, Maguire KC, Mersey B, & Ferrer EA (2011). Has the Earth’s sixth mass extinction already arrived?. Nature, 471 (7336), 51-7 PMID: 21368823
4. van Heezik, Y., Smyth, A., Adams, A., & Gordon, J. (2010). Do domestic cats impose an unsustainable harvest on urban bird populations?. Biological Conservation, 143 (1), 121-130 DOI: 10.1016/j.biocon.2009.09.013
5. Liker A, Papp Z, Bókony V, & Lendvai AZ (2008). Lean birds in the city: body size and condition of house sparrows along the urbanization gradient. The Journal of animal ecology, 77 (4), 789-95 PMID: 18479344
6. Miller, M. (2006). Apparent Effects of Light Pollution on Singing Behavior of American Robins. The Condor, 108 (1) DOI: 10.1650/0010-5422(2006)108[0130:AEOLPO]2.0.CO;2
7. Slabbekoorn, H., & Peet, M. (2003). Ecology: Birds sing at a higher pitch in urban noise. Nature, 424 (6946), 267-267 DOI: 10.1038/424267a
8. Baker, M., Bjerke, T., Lampe, H., & Espmark, Y. (1986). Sexual Response of Female Great Tits to Variation in Size of Males’ Song Repertoires. The American Naturalist, 128 (4) DOI: 10.1086/284582
9. Krebs, J., Ashcroft, R., & Webber, M. (1978). Song repertoires and territory defence in the great tit. Nature, 271 (5645), 539-542 DOI: 10.1038/271539a0
10. Nemeth, E., & Brumm, H. (2009). Blackbirds sing higher-pitched songs in cities: adaptation to habitat acoustics or side-effect of urbanization? Animal Behaviour, 78 (3), 637-641 DOI: 10.1016/j.anbehav.2009.06.016
11. McLaughlin, K., & Kunc, H. (2012). Experimentally increased noise levels change spatial and singing behaviour. Biology Letters DOI: 10.1098/rsbl.2012.0771
12. Wood, W., & Yezerinac, S. (2006). Song sparrow (Melospiza melodia) song varies with urban noise. The Auk, 123 (3) DOI: 10.1642/0004-8038(2006)123[650:SSMMSV]2.0.CO;2
13. Mockford, E., & Marshall, R. (2009). Effects of urban noise on song and response behaviour in great tits. Proceedings of the Royal Society B: Biological Sciences, 276 (1669), 2979-2985 DOI: 10.1098/rspb.2009.0586
14. Mockford, E., Marshall, R., & Dabelsteen, T. (2011). Degradation of Rural and Urban Great Tit Song: Testing Transmission Efficiency. PLoS ONE, 6 (12) DOI: 10.1371/journal.pone.0028242
15. Brumm, H., & Slabbekoorn, H. (2005). Acoustic communication in noise. Advances in the Study of Behavior, 35, 151-209 DOI: 10.1016/S0065-3454(05)35004-2
16. Slabbekoorn H, & Smith TB (2002). Habitat-dependent song divergence in the little greenbul: an analysis of environmental selection pressures on acoustic signals. Evolution; international journal of organic evolution, 56 (9), 1849-58 PMID: 12389730
17. Nemeth, E., Pieretti, N., Zollinger, S., Geberzahn, N., Partecke, J., Miranda, A., & Brumm, H. (2013). Bird song and anthropogenic noise: vocal constraints may explain why birds sing higher-frequency songs in cities. Proceedings of the Royal Society B: Biological Sciences, 280 (1754), 20122798-20122798 DOI: 10.1098/rspb.2012.2798
18. Nemeth, E., & Brumm, H. (2010). Birds and Anthropogenic Noise: Are Urban Songs Adaptive?. The American Naturalist, 176 (4), 465-475 DOI: 10.1086/656275
19. Schuster, S., Zollinger, S., Lesku, J., & Brumm, H. (2012). On the evolution of noise-dependent vocal plasticity in birds. Biology Letters, 8 (6), 913-916 DOI: 10.1098/rsbl.2012.0676
20. Osmanski, M., & Dooling, R. (2009). The effect of altered auditory feedback on control of vocal production in budgerigars (Melopsittacus undulatus). The Journal of the Acoustical Society of America, 126 (2) DOI: 10.1121/1.3158928
21. Brumm, H. (2004). The impact of environmental noise on song amplitude in a territorial bird. Journal of Animal Ecology, 73 (3), 434-440 DOI: 10.1111/j.0021-8790.2004.00814.x
22. Titze, I. R. (1994). Principles of voice production (pp. 279-306). Englewood Cliffs: Prentice Hall.
23. Cynx J, Lewis R, Tavel B, & Tse H (1998). Amplitude regulation of vocalizations in noise by a songbird, Taeniopygia guttata. Animal behaviour, 56 (1), 107-13 PMID: 9710467
24. Elemans, C., Zaccarelli, R., & Herzel, H. (2008). Biomechanics and control of vocalization in a non-songbird Journal of The Royal Society Interface, 5 (24), 691-703 DOI: 10.1098/rsif.2007.1237
















![Origin_of_Species_title_page[1]](https://ecologicablog.files.wordpress.com/2013/05/origin_of_species_title_page1.jpg?w=224&h=351)
![The_Selfish_Gene3[1]](https://ecologicablog.files.wordpress.com/2013/05/the_selfish_gene31.jpg?w=219&h=333)
![125286[1]](https://ecologicablog.files.wordpress.com/2013/05/1252861.jpg?w=232&h=355)

![dn16347-1_300[1]](https://ecologicablog.files.wordpress.com/2013/05/dn16347-1_3001.jpg?w=267&h=407)










